All you need to know about humidity test chambers
Definition

What is a humidity test chamber?
A humidity test chamber, also known as a climate cabinet or climate chamber, is a unit used to simulate certain environmental conditions (temperature and relative humidity). Environmental simulation testing in climate chambers provides an indication of how test specimens will behave under defined climate conditions. The tests therefore ensure product quality and stability and give an idea as to their service life and durability. It is important that the units ensure homogeneous climate conditions in terms of time and space and that the climate conditions are documented continuously during the tests (data log functions for the temperature and humidity process parameters).
Structure
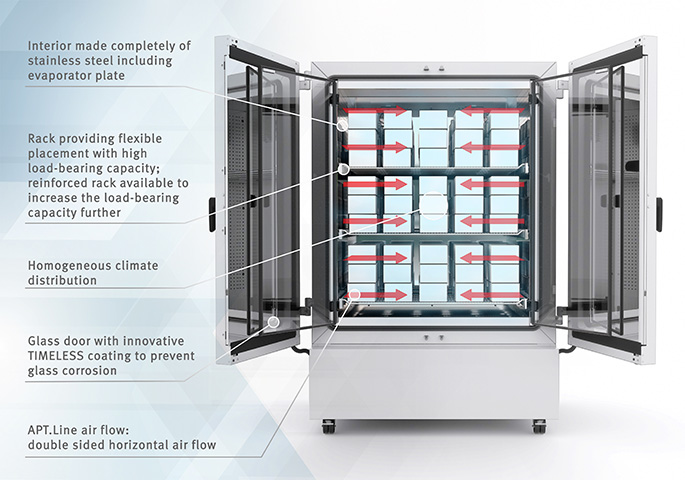
Structure and function of a humidity test chamber
A humidity test chamber features an interior (usable space) in which the required climate conditions (temperature and relative humidity) are generated and maintained over a predefined period of time.
The outstanding climate homogeneity in BINDER climate chambers is a result of the doubled-sided horizontal air flow. The air is sucked toward the back into the rear wall, which is perforated. In the rear of the chamber, which is not part of the usable space, the air is reconditioned and therefore adjusted to the set temperature and humidity before it is directed to the sides and back into the usable space. This double-sided horizontal air flow creates the particularly constant and homogeneous climate in BINDER units.
Humidity test chamber applications
Humidity test chambers are used in many different sectors and industries, wherever stability, shelf-life, and aging tests are required.

ICH stability testing
Drug manufacturers need to specify an expiration date for their products as part of the approval process. This expiration date must be determined experimentally with stability studies. In the humidity test chamber, the drugs are stored under defined climatic conditions (temperature and relative humidity). The storage conditions are defined in the guidelines from the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) and must be maintained precisely. BINDER climate chambers fulfill all the requirements of the ICH guidelines and provide the ideal solutions for stability testing for pharmaceutical active ingredients and products. BINDER humidity test chambers are also suitable for performing long-term stability testing, as well as accelerated tests and stress tests in line with the ICH guidelines. The process parameters can be documented in a tamper-proof way using the network-enabled BINDER APT-COM software. Documentation is in line with GLP/GMP guidelines, which meet the requirements of FDA Regulation 21 CFR 11.

Development of plant-based meat alternatives
Consumers want plant-based meat alternatives to look and taste just like real meat and therefore also have the same texture. The food-tech startup Planted, which makes plant-based meat from proteins such as peas, sunflowers, and oats, uses BINDER humidity test chambers for product development.
Fermentation plays a crucial role when developing plant-based meat products. Humidity test chambers help find the way to optimum fermentation and ultimately to the perfect product. The climate chamber used for this must be capable of handling a wide range of temperatures and humidity levels. The microorganisms are fermented in the chamber and selected based on the results. In the BINDER KMF humidity test chamber, the fermentation parameters can be precisely set and controlled in a broad temperature and humidity range.
Planted also uses the humidity test chambers for processes other than fermentation to test what influence heat has on the taste and texture of the plant-based meat products. They are also used to determine the shelf life and storage conditions.
Aging tests in medical technology
Surgical instruments need to meet the very highest quality standards and be checked extensively before they can be used for human or veterinary medicine, or in dentistry. Humidity test chambers are also used to perform aging tests. Aging tests are carried out in two different ways: in real time and accelerated. Accelerated aging tests are used to simulate the real-time aging of medical devices at shorter timescales. The products are stored in the climate chamber under defined climate conditions (temperature and relative humidity) over a certain period of time. These conditions are defined in the testing standard ASTM F1980 (Standard Guide for Accelerated Aging of Sterile Medical Devices).

Research
At the Fraunhofer Institute for Casting, Composite, and Processing Technology (IGCV), Siegfried Bähr used a BINDER climate chamber to investigate how metallic powder materials interact with ambient humidity for laser beam melting for his master’s thesis. He subjected different powder materials to a range of climate conditions and observed the increase and decrease in moisture.
He also wanted to uncover whether there was any link between the moisture in the powder material in its original state and the quality of the component later down the line.
As part of the experiments, he also placed the powder samples in an aluminum dish, which was then placed on a set of scales inside the chamber. The scales were positioned on an anti-vibration base plate. By recording the mass at certain intervals under a range of climate conditions, Bähr was able to dynamically record the increase in moisture in the powder.
Standards for working with humidity test chambers
The following guidelines govern the applications carried out in climate chambers:
- ICH Q1A (R2), ICH Q1B, ICH Q5C, VICH for stability testing of active ingredients and finished drugs
- Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) in the chemical, pharmaceutical, and life sciences industries
- ASTM F1980 (Standard Guide for Accelerated Aging of Sterile Medical Devices)
In the pharmaceutical industry, the requirements of Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) are described in FDA Regulation 21 CFR Part 11. They regulate how to handle measuring instruments, which must be calibrated and tested at specific intervals. A data logger must record all test parameters and forward them to the software and the storage medium, where they are processed and archived. This FDA-compliant documentation can be ensured with the network-enabled BINDER APT-COM software.
You will find everything you need to know about GLP in our whitepaper: “Good Laboratory Practice” – What is behind all this?”
You can read all about ICH stability testing and how to choose the right climate chamber for this in our whitepaper “Stability testing according to ICH Q1A (R2): Basics and technical solutions.”
From what point is a constant climate chamber ready for use?
In total, the climate chamber goes through three steps during validation:
Selection criteria when buying a humidity test chamber
There are two types of units: compressor-based units, which work with refrigerants, and thermoelectric cooling-based units, which heat and cool thermoelectrically. Each of these technologies meets different requirements and is therefore suitable for different applications. Thermoelectric cooling-based units are designed for long-term and accelerated ICH stability testing, while compressor-based units are also suitable for stress tests. Climate chambers with thermoelectric cooling are more environmentally friendly and quieter. They also use significantly less energy than models with a compressor. Which technology is most suitable always depends on the needs of the users and which application is required.
The air movement in a humidity test chamber is crucial for climate homogeneity inside the unit. Only homogeneous climate conditions, both in terms of space and time, provide reliable results. Double-sided horizontal air flow allows for particularly homogeneous conditions. The air is extracted via a perforated rear wall and returned back into the usable space after reconditioning. The air is distributed evenly into the chamber via small perforations in the side walls and therefore flows evenly over the test materials from both sides.
- Type of water supply and disposal:
The water can be supplied and disposed of via a connection to the on-site water supply or via large-volume water canisters for fresh water supply and collecting condensed water that are mounted directly on the climate chamber. - Type of humidification:
The most common types of humidification are steam and ultrasonic humidification systems. Climate chambers from BINDER feature steam humidification systems, as these ensure quick and accurate humidification and this technology prevents condensation in the interior. - Water quality:
Fully desalinated water with a conductivity between 1 µS/cm and max. 20 µS/cm is the prerequisite for reliable continuous operation of the unit. If the water quality is inadequate, the tap water must be treated. The BINDER PURE AQUA SERVICE water treatment system can be used here as an accessory, both when using via the mains water connection and when the units are supplied via canisters.
- As many components as possible in the unit should be made from corrosion-resistant stainless steel.
- A triple door gasket guarantees maximum reliability.
- Long-life steam humidifiers have been proven to have a very low failure rate of less than one percent in the first five years of continuous operation.
Humidity test chambers for every application
Models
Humidity test chambers are available in different sizes and with different temperature and humidity ranges. Before purchasing, you need to consider in detail the applications for which you need the unit. In every case, a humidity test chamber should always guarantee reliable long-term operation and be able to maintain the set climate conditions precisely.
The double-sided horizontal air flow ensures precise and constant climate homogeneity both in terms of space and time in all BINDER climate chambers – even over long periods of time and when the chamber is fully loaded.
With its large temperature and humidity range from 0 °C to +70 °C and 10 % to 80 % RH, the KBF series is ideal for all test procedures in the pharmaceutical industry according to ICH Q1A: long-term stability testing, accelerated stability testing, and also stress tests, where particularly high temperatures and humidity levels are used.
The KBF P series is also equipped with an ICH-compliant light source, making it the perfect solution for photostability tests according to ICH guideline Q1B. The main advantage of the KBF LQC series is the patented light photometry for photostability tests. Two 3D spherical sensors control the light dosage of UV-A and visible light. Once the desired light dosage is achieved, the chamber switches the illumination off automatically.
The thermoelectric cooling-based KBF-S ECO series provides a particularly environmentally friendly and energy-saving solution for stability testing. Thanks to thermoelectric heating and cooling, refrigerant is not required and the annual energy consumption is up to 75 percent lower than with compressor-based units. It is possible to carry out particularly energy-saving long-term stability testing and accelerated stability testing according to ICH Q1A in units from the KBF-S ECO series.
Demanding material tests, typically at 85 °C and 85 % relative humidity (THB test = temperature humidity bias), are no problem with the KMF series with expanded temperature / humidity range of -10 °C to +100 °C and 10 % to 98 % RH.
The humidity test chambers in the KBF and KBF PRO series are operated with climate-neutral refrigerant and consume particularly little energy. You can use the energy savings calculator to easily work out how much energy you can save.
With the ICH light modules, appliances from the KBF and KBF PRO series can be transformed into photostability test chambers in no time at all.
KBF series
in sizes of 130, 260, 470, 720, 1060 and 1600 liters
KBF PRO series
in sizes of 130, 260, 470, 720, 1060 and 1600 liters
KBF-S ECO series
in sizes of 240, 400, 720 and 1020 liters
Find the right humidity test chamber for your application
Humidity test chambers simulate temperature and humidity and are mainly used for environmental simulation tests to determine the service life and durability of products, materials, etc. They are used in different sectors, such as the pharmaceutical industry, the food industry, and the cosmetics industry, as well as in research.
Humidity test chambers play a key role in the execution of ICH stability testing, which is an important prerequisite for approving drugs. For this, the units must maintain the predefined climate parameters exactly and ensure smooth, reliable continuous operation. Important factors when choosing a humidity test chamber are heating and cooling technology, air flow, water management, and light.
You can read more in our whitepaper “Stability testing according to ICH Q1A (R2): Basics and technical solutions.”
BINDER offers a broad range of climate chambers for different types of environmental simulation tests, from long-term stability testing and accelerated shelf-life testing right through to demanding stress tests. Find the right unit for your application. We would be happy to advise you in this regard.


