With an interior volume of 12 cubic meters
WIC 1 model
Go to product
A walk-in chamber is a climate test chamber with large dimensions that users are able to enter. A walk-in chamber, just like a constant climate chamber, simulates specific environmental conditions (temperature and relative humidity). Environmental simulation testing in walk-in chambers indicates how test specimens will behave under specific climate conditions. This testing plays a key role in product quality assurance and also helps to determine the service life and durability of the products. The crucial factor is that walk-in chambers ensure homogeneous climate conditions in terms of both time and space.
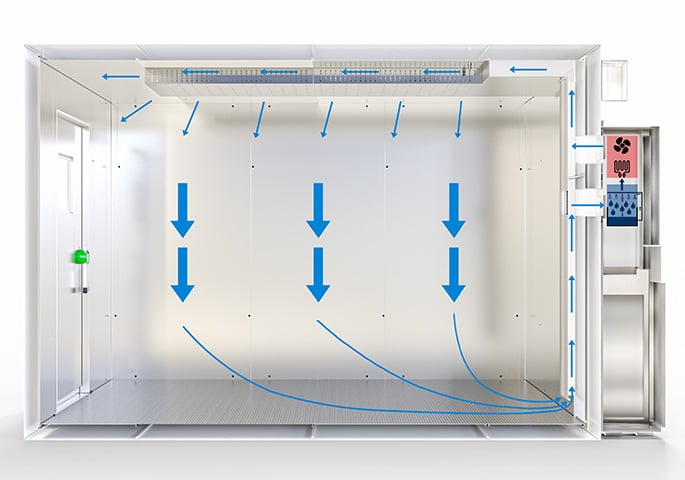
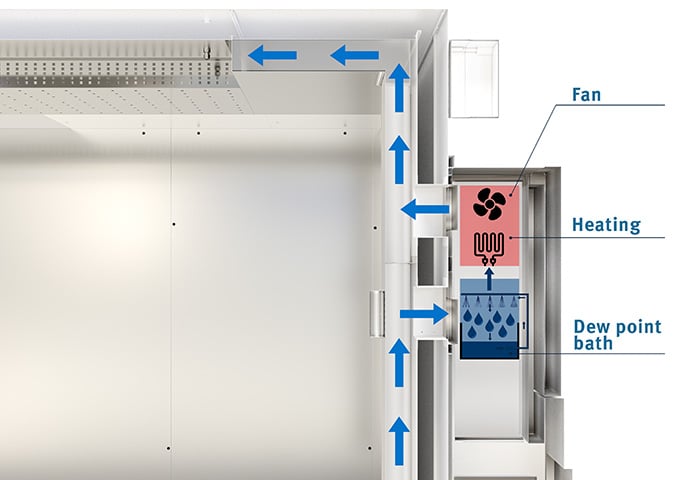
Just like a constant climate chamber, a walk-in chamber features an airtight interior in which the required climate conditions (temperature and relative humidity) are generated and maintained over a predefined period of time.
On BINDER walk-in chambers, the unit that generates and maintains the required climate conditions is installed on the outside of the climate chamber. This makes it easier to perform service work and reduces any disruption to the climatic conditions in the interior to a minimum.
Walk-in chambers are primarily used in the fields of pharmaceuticals, medical technology, food, and cosmetics. They are used whenever stability, shelf-life, and aging tests are required. Walk-in chambers provide much more space compared to traditional reach-in climate chambers and can be a good alternative when testing or storing large quantities or bulky specimens in particular.
Stability testing is used to determine the expiration dates of drugs based on experiments. To this end, the products are stored in climate chambers under defined climatic conditions (temperature and humidity). The storage conditions are defined in guidelines from the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) and must be strictly adhered to. If the door is opened in order to load or remove specimens, the climate chamber must be able to restore the specified climatic conditions within two hours.
BINDER walk-in chambers are ideal for long-term and accelerated stability testing of active pharmaceutical ingredients and products in accordance with the ICH Q1A (R2) and VICH GL3 guidelines.
Surgical instruments need to meet the very highest quality standards and be checked extensively before they can be used in human or veterinary medicine, or in dentistry. Walk-in chambers are used here too – for example, in order to perform aging tests. Aging tests are carried out in two different ways: in real time and accelerated. Accelerated aging tests are used to simulate the real-time aging of medical devices. The devices are stored in the walk-in chamber under defined climate conditions (temperature and relative humidity) over a certain period of time. These conditions are defined in the testing standard ASTM F1980 (Standard Guide for Accelerated Aging of Sterile Barrier Systems and Medical Devices).

In the food industry, shelf-life testing is carried out to determine the shelf life of a product under different storage conditions and therefore calculate the best-before date. Accelerated shelf-life testing (ASLT) is used to obtain meaningful results quickly. It involves using a walk-in chamber to simulate temperature, relative humidity, and light. The samples are stored at different temperatures, such as 20°C, 30°C, and 40°C, and exposed to a high level of light intensity. Finally, color loss is measured as changes in hue (ΔH) as a function of different time intervals. Chemical, microbiological, and physical changes are measured at specific time intervals until the product is no longer edible.

Cosmetics undergo stability testing in order to determine how the formulation and packaging of the products behave under specific climatic conditions. The aim is to meet the necessary physical, chemical, and microbial quality standards and to ensure the integrity of the products and packaging in order to guarantee the shelf life and safety of cosmetic products. Stability tests are required for every new cosmetic product that is placed on the market and must also be carried out in the event of changes to the manufacturing process, packaging, or selection of raw materials. The tests are performed in accordance with international regulations and standards such as ISO/TR 18811 (Guidelines on the stability testing of cosmetic products) and the guidelines issued by Cosmetics Europe (formerly Colipa). Stability testing is generally carried out under “accelerated” conditions. The products are exposed to higher temperatures, higher humidity, and intense light in order to estimate the shelf life.
Constant climate chambers are used in microbiological applications in order to cultivate bacteria. Controlled high humidity prevents the culture dishes from drying out in this case. Climate chambers can also be used to breed insects or plants. In these applications, particular attention must be paid to the required air change rate and the need for light, especially when cultivating plants. Both the ventilation and the heat input from the illumination pose significant challenges with regard to maintaining the climate. Depending on the selected climate point (combination of temperature and humidity), BINDER walk-in chambers can provide air change rates of approx. 20 m3/h or heat compensation of approx. 500 W.
The following guidelines govern the applications carried out in walk-in chambers:
In the pharmaceutical industry, the requirements of Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) are described in FDA Regulation 21 CFR Part 11. They regulate how to handle measuring instruments, which should be calibrated and tested at specific intervals. A data logger must record all test parameters and forward them to the software and the storage medium, where they are processed and archived. The software itself must also meet high standards as defined in 21 CFR Part 11.
With the aid of the optional monitoring system in combination with the BINDER APT-COM software, the climate parameters of BINDER walk-in chambers can be documented in an FDA-compliant manner.
You will find everything you need to know about GLP in our whitepaper: “Good Laboratory Practice” – What is behind all this?
Installation and qualification for BINDER walk-in chambers:
In total, the walk-in chamber goes through three steps during validation:
Walk-in chambers from BINDER are available in three different sizes:
Shelving sets in three different sizes are available as accessories for WIC constant climate chambers. The shelving sets feature 4 levels as standard and provide storage space of 12 m2 (WIC1), 17 m2 (WIC2), or 22 m2 (WIC3). Additional shelves (up to 11 levels) can be added if necessary.
A walk-in chamber takes up significantly more space than a conventional climate chamber. Excluding fittings and connections, the chambers have a width of 2.6 meters, a height of 2.47 meters, and – depending on the model – a length of 2.5, 3.6, or 4.7 meters. In addition, the external air conditioning unit with a footprint of 36.2 x 58.6 cm and a maintenance space with a width of at least 50 cm must also be taken into account. Alternatively, the air conditioning unit can be positioned next to the door.
The walk-in chamber is assembled on site and the individual components are delivered on pallets. Delivery is to the curbside as standard; on-site transportation to the point of use is to be agreed with the customer on an individual basis.
All key information regarding installation can be found in the Pre-Installation Checklist.
BINDER walk-in chambers from the WIC series are available in three different sizes with an interior area of six, nine, or twelve square meters. With a temperature range of 10°C to 50°C (temperature accuracy of ± 1.5°C) and relative humidity of 20% to 90% (relative humidity accuracy of ± 2.5%), they are perfect for long-term and accelerated stability testing in accordance with ICH guideline Q1A (R2).
The standard equipment includes:
Available as accessories:
The walk-in chambers have a delivery time of eight to ten weeks.
You can find useful information on the installation requirements in our checklist:
Walk-in chambers simulate temperature and humidity and are used for stability, shelf-life, and aging tests. They serve as an alternative to a conventional constant climate chamber – particularly when testing large quantities or bulky specimens.
BINDER walk-in chambers are ideal for ICH long-term and accelerated stability testing because they maintain the specified climate parameters precisely in terms of both time and space and ensure reliable, fault-free long-term operation. Both the interior and the heavy-duty shelving (available as an accessory) are made entirely from stainless steel. This ensures that BINDER walk-in chambers are durable products that can meet even the stringent requirements of the pharmaceutical industry. When it comes to choosing the right walk-in chamber, the footprint and spatial requirements are important factors that must be taken into account before buying.
The walk-in chambers have a flexible design and are tailored to customers’ needs – for example, the positioning of the door and the external air conditioning unit can be selected based on the installation conditions.
Alongside the standard equipment, there is a wide range of accessories available to turn any chamber into a custom-made solution.